Although it’s popularly regarded as an enemy to our health, in the right proportions fat is crucial for our bodies to function. The stuff stores energy in the form of lipid droplets, which are normally located near the mitochondria – the internal cellular structures that convert oxygen into useable chemical energy. When other nutrients run low, the mitochondria turn to lipids as an alternative fuel source.
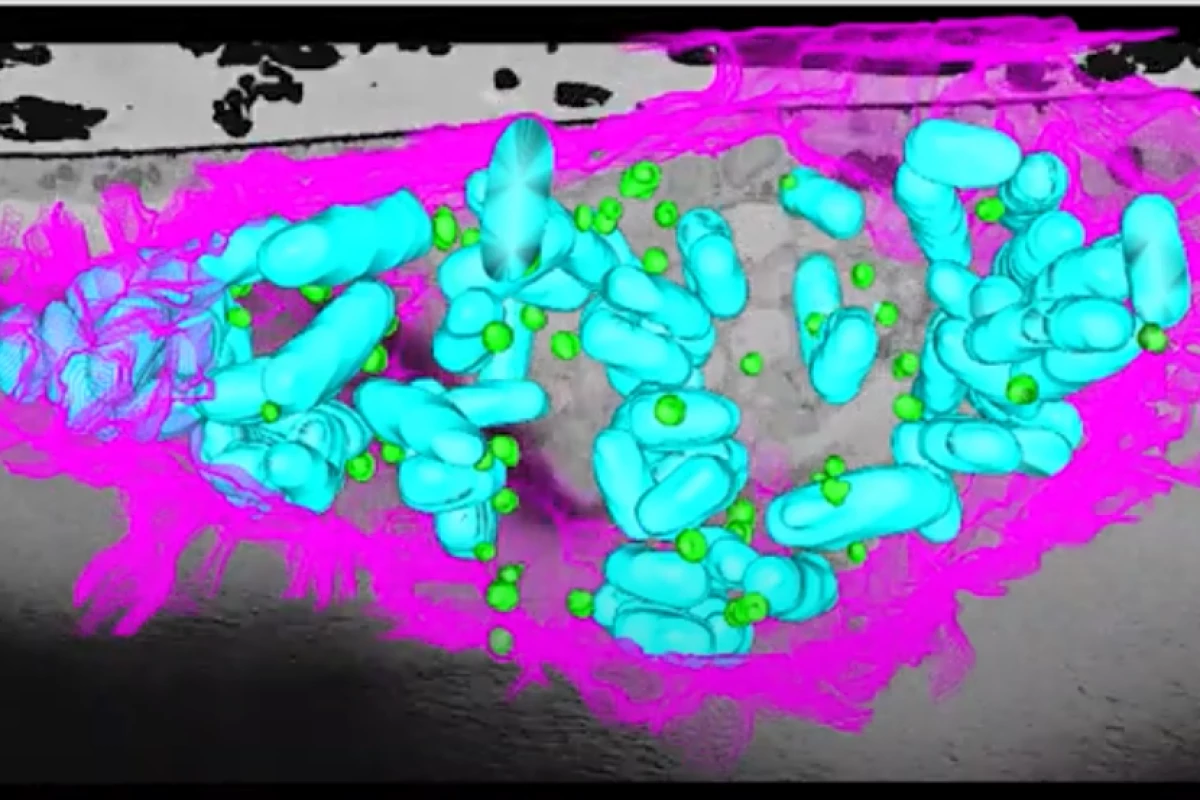
But now a new study has found a surprising new mechanism. When a cell is invaded by bacteria, lipids migrate from the mitochondria to the parts of the cell where bacteria are present. The attackers often eat the energy-rich lipids; and now it turns out that cells may use this to their advantage, by "poisoning" the lipids before serving them up to the bacteria.
"Fat is part of the cell’s arsenal – cells manufacture toxic proteins, package them into the lipid droplets, then fire them at the intruders", says Robert Parton, an author of the study.
The team says that these lipid droplets essentially form a first-line of defense against infection inside cells. In so doing, they actually change the metabolism of the cell.
This process had previously been identified in the cells of fruit flies, so for the new study the team investigated whether it also applied to mammalian cells. Electron microscope observations of macrophages (white blood cells) in humans and mice showed that it did. The discovery could lead to new techniques against bacteria that are developing resistance to antibiotics.
The team outlines the work in the video below, "Fats fighting back against bacteria".
"Our next step is to find out how the lipid droplets target the bacteria. By understanding the body’s natural defenses, we can develop new therapies that don’t rely on antibiotics to fight drug-resistant infections", says Parton.