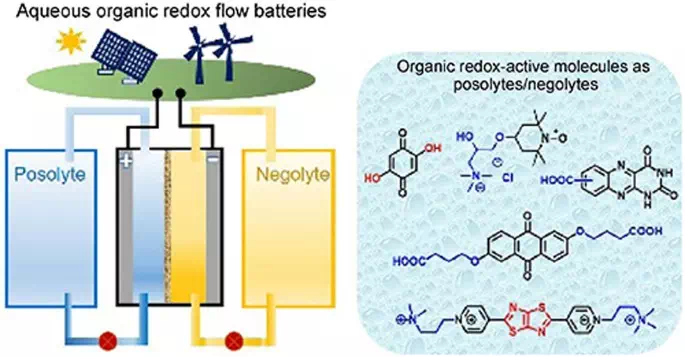
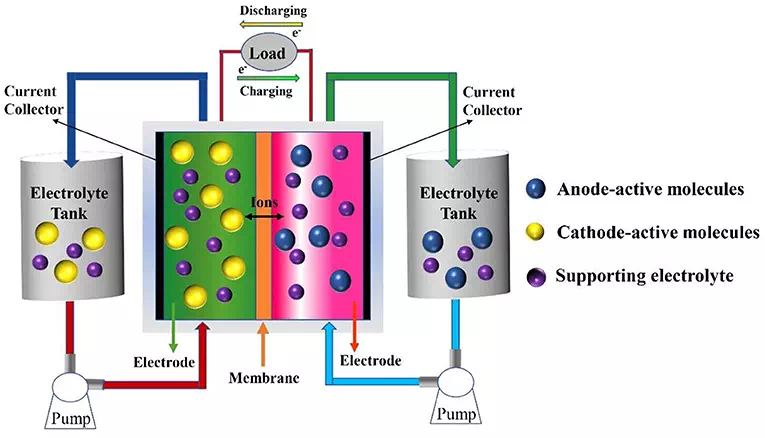
Redox flow batteries can store vast amounts of energy at relatively low cost. Redox flow batteries can store energy in liquid electrolytes housed in huge external tanks for months at a time.
Something that doesn’t help the eco-credentials of redox flow batteries, however, is their use of a scarce and expensive metal known as vanadium. This metal is the basis of the electrolyte solution and offers great reliability during charging and discharging, but some researchers see a greener alternative in water-based electrolytes.
Another area with room for improvement is the electrodes of redox flow batteries, which are typically made from a synthetic polymer called carbonized polyacrylonitrile. But the Linköping University team is putting forward another solution while solving the electrolyte issue at the same time, producing what it bills as the first all-organic redox flow battery.
The team’s redox flow battery features electrodes made from PEDOT, which is an organic and conducing polymer we’ve also seen used in advanced lithium-ion battery designs, and even "smart bricks" that store energy. The engineers doped their PEDOT polymer to enable it to transport the battery's positive and negative ions, and work nicely with a water-based electrolyte laden with quinone molecules, which occur naturally in forest-based materials.
"Quinones can be derived from wood, but here we have used the same molecule, together with different variants of the conducting polymer PEDOT" says study author Viktor Gueskine. It turns out that they are highly compatible with each other.
The team found that the PEDOT electrodes and quinone-based electrolytes worked together to promote the flow of protons and electrons in the battery.
The device is very cheap, recyclable and perfectly safe, opening up the possibility of installing such a battery in homes to act as a power bank for electric vehicles.